Criteria for the on-target black coloration of concrete products
Bringing added value to a concrete product by means of suitable coloration and emphasizing not only its functionality but also its aesthetic aspects paves the way to higher product prices and additionally offers yet another opportunity to stand out in the competition among suppliers by making a powerful color statement.
In recent years, there has been a growing impression that one important main color dominates the coloration of concrete products: “Black concrete is beautiful.” It is of particular interest to the manufacturers of black products that there is a basic choice of two types of pigments for black coloring: black iron oxides and carbon pigments. Black coloring is therefore an absolute exception. Characteristic properties of the carbon pigments offer additional possibilities in the black range which can be made use of in the coloring process.
Different pigments for black coloring
To ensure compliance with the different concrete-specific and quality-specific requirements, it is prudent to use quality-controlled pigments according to DIN EN 12 878 from a fully certified manufacturer only. Carbon and iron oxide pigments come in all standard preparations such as powders, liquids and granules. Application-related aspects with regard to the different types of pigments and their merits and drawbacks, also in combination with the dosage system to be used, have been described in a number of publications ([1] to [6]) and are therefore not the focus of this article. The objective here is to present the properties and modes of action of the black pigments and to define which pigment quality is most beneficial and suitable for the task at hand. The article also deals with the limitations of the different types of pigments in order to assist the user in choosing the right pigment and enable a more specific use of the pigments in the black coloration of concrete products.
Achieving the black color in concrete products
Strictly speaking, black concrete could be produced only if the concrete surface fully absorbed the incident light, meaning that a person looking at the concrete surface were then unable to perceive reflected or scattered light. This means that only those substances are suitable for black coloring which are able to absorb the light preferably across the entire visible spectrum and reflect or scatter the light to a very limited extent. Colored pigments, on the other hand, are intended to absorb the light selectively and reflect a specific color contribution. Considering the purely optical properties of black pigments as the single criterion, it can be said that the optical performance of carbon pigments is superior to that of iron oxides [7]. The absorption rate of carbons can be as high as 99.8 % [12].
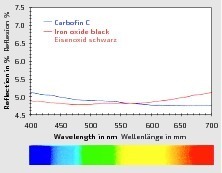
When comparing the reflective properties in terms of wavelength, carbons (Carbofin C) reflect the light more strongly than iron oxides in the blue range of the color spectrum. Iron oxides, on the other hand, reflect the light much more strongly in the red/orange range of the color spectrum (Fig. 2). These optical differences also explain why carbons enable blue-nuanced color results to be achieved whereas, in comparison, iron oxides often appear slightly yellow to yellow-brownish in shade.
In addition to the color contributed by the non-colored concrete product, the physical absorption and reflective properties of the pigment used for coloring are therefore a decisive factor for design purposes.
Color effect of black pigments
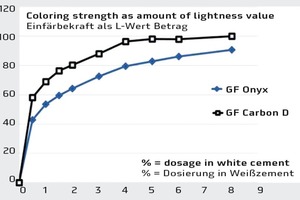
Cement-bound building materials are colored by means of “coating” or “covering” the calcium silicate hydrate structures of the cement paste forming. The more complete the coverage, the more intense is the effect of the optical characteristics of the pigments described above. It is logical therefore that the coloring strength increases when increasing the quantities added (Fig. 3 and 4). In practical use, however, iron oxide and carbon pigments are found to differ greatly in terms of their color shades and coloring strength.
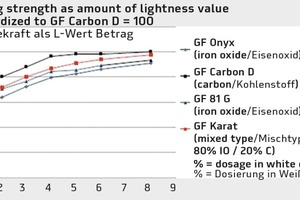
When analyzing the coloring strength colorimetrically, a characteristic coloring curve results for each pigment preparation. Figure 4 shows the curves for Granufin Onyx, an iron oxide, and for the coarse-particle Granufin Carbon D carbon pigment.
Both the image of the laboratory prisms (Fig. 3) and the diagram (Fig. 4) clearly show that the color nuances and color depth of carbons cannot be achieved with classic iron oxides even at unusually high dosages of 8 %. The graph also shows that the coloring strength of an added quantity of 4 % of iron oxide pigment (GF Onyx) can already be achieved by adding slightly less than 2 % of the GF Carbon D carbon pigment.
Properties of carbon pigments
As with the optical properties, basic physical properties such as the differences in density and size of the pigment particles are the reasons that carbon pigments are very economical in use compared with iron oxide blackeners.
At approximately 4.6 g/cm³, the pigment density of iron oxides is nearly three times higher than that of carbon pigments at approximately 1.8 g/cm³. The immediate conclusion from this is that, with a comparable pigment particle diameter, nearly three times the volume of coloring particles per kg of pigment is bought when selecting the carbon pigment which is beneficial in terms of density.
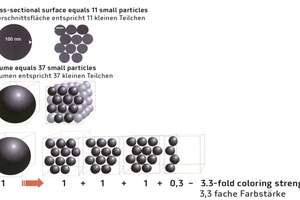
Particle size is another decisive factor for the coloring strength. The more the cement paste is covered by the light-absorbing pigment surface, the blacker the concrete product appears to be. The influence of the particle size can be modelled quite easily.
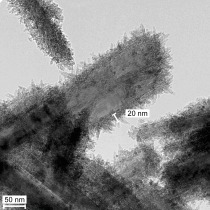
Consider a spherical pigment particle with a diameter of 100 nm (typical mean particle size of a coarser-particle carbon preparation). When coating the cement paste, the absorbing action of the pigment particle is limited to the cross-sectional surface of the sphere. When modelling 37 smaller spheres with a diameter of 30 nm each (typical mean particle size of a fine-particle cement blackener), the spherical volume of all of the smaller spheres precisely equals the volume of the large sphere having a diameter of 100 nm. In the model, optimal distribution of the small spheres on the calcium hydrate needles of the cement paste matrix by means of “dispersion”, however, results in 3.3 times the pigment surface available for “absorption”, that is, coloration (Fig. 5).
Finer-particle pigments, when well dispersed or distributed, enable more cement paste to be covered and therefore additional coloring strength to be achieved based on the same initial pigment weight. It should be noted, however, that the optical characteristics of finer-particle pigment preparations can result in an increasing yellow component in the natural color due to light refraction and diffraction effects.
The particle size of the pigments is of central importance in terms of resistance to weathering as smaller particle sizes can result in resistance problems due to the limited fixation of the color particles into the concrete matrix.
Criteria for the resistance of the black pigments
The basic question to be considered prior to selecting a pigment suitable for black coloration is the resistance of the pigment in the concrete product to be produced. Both iron oxides and carbon pigments offer excellent resistance to alkali and ultraviolet radiation. Anchoring the pigments in the concrete matrix is crucial for color permanency. Fixation of the pigments can only occur in the calcium silicate hydrate matrix which forms from the cement clinker. These matted hydrate needles not only promote strength but also bind the pigment particles into the concrete. This results in a concrete product exhibiting a pore structure that contains both gel and capillary pores.
Two factors of success are thus crucial in binding the pigments into the concrete mechanically: the size of the capillary pores forming on the one hand, and the size of the pigment particles on the other. It is therefore very important that coarse-particle pigment preparations are used to the greatest possible extent. They must contain a sufficient amount of pigment particles larger than the pore structure of the concrete. When this is the case, the pigments are fixed mechanically and can thus not escape or be washed out from the matrix grid of the cement paste forming. This is of importance in particular for carbons because different manufacturing processes, each with a different particle size distribution, are available as raw materials for these types of pigments. The particle size distribution in the carbon pigment interacting with the pore structure is the reason for recommending a minimum added quantity of around 3 %. This reserve dosage is to ensure that even in the event of a certain amount of weathering in a portion of the fine-particle carbon particles the coarser-particle carbon pigments remaining in the product maintain the black coloration.
Pore structure an essential element of pigment fixation
The pore structure of the concrete is an essential element of pigment fixation. Being the sole responsibility of the concrete manufacturer, it is exposed to a number of influencing factors. According to literature, the pore diameter in concrete ranges from 10 nm to 0.1 mm. There is a strong correlation between the water-cement ratio (w/c ratio) and pore diameter. At low w/c ratios and good hydration, the capillary pores have a diameter of 10 nm to 50 nm; at a w/c ratio of 0.4, the diameter increases to approximately 100 nm. Higher w/c ratios result in a significant increase of the pore diameters as can be verified by means of penetration tests [8].
It is a known fact that, with exposed concrete or precast concrete, the cement paste layer sets more poorly at the interfaces between concrete and formwork, where higher local w/c ratios may occur as a result of excess water, than is the case with typical no-slump concretes in the production of concrete paving blocks. It is therefore recommended to use mainly iron oxides for coloring exposed and precast concretes as these are also fixed electrostatically.
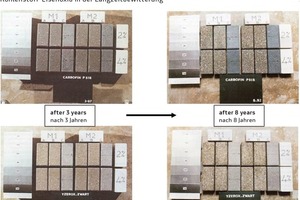
The excellent long-term weathering results achieved when using high-quality carbon pigments for the production of concrete paving blocks have been confirmed by numerous field results (cf. [9-11]), as is also shown in a comparative example (Fig. 8).
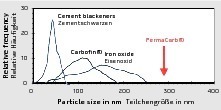
Even though the performance has been widely proven, a long-term weathering test is required to clarify whether a specific carbon quality is suitable for coloration in the customer’s system because of the multitude of differences in each concrete mixture and the concrete structure forming under the given production conditions. The field test is crucial also because there is a larger choice of different raw carbon qualities available in the market. For the production of coarser-particle, weathering-resistant facemix qualities, different carbons are available as raw materials with particle sizes ranging from approx. 70 nm to 290 nm. The size of the primary particles in carbons ranges from approx. 5 nm to 500 nm while black iron oxide pigments have a diameter of between 100 nm and 600 nm. Corresponding raw material qualities and particle size distributions are depicted in Figures 6 and 7.
Usability of fine-particle cement blackeners
Due to their excellent coloring strength, fine-particle, color-intensive cement blackeners (mean particle size approx. 30 nm) are suitable mainly for the effective, low-cost coloring of concrete cores. The resulting concrete products are then prone to weathering, however, so that the concrete products colored in this way should be fully built-in after placing.
Mechanical binding is excellent as the mean particle size of iron oxides is usually significantly larger than the pore structure of the concrete block to be covered. Electrostatic fixation into the concrete block is additionally also possible. The suitability of iron oxides for coloring concrete is therefore standard practice and well established. In contrast to carbon pigments, there is consequently no recommended minimum dosage.
Iron oxide pigments additionally allow multiple mixed color shades to be produced by means of additive weighing. In this application, the exclusive use of iron oxides as an additive black component is recommended as they, like the remaining color shades, bind into the concrete structure as iron oxides do, that is, uniformly and thus in a comparable physical manner. It is strongly recommended not to use carbon pigments, including coarse-particle ones, for the mixing of brown color shades.
New developments of advanced iron oxides
If iron oxides are to be used to achieve a deep-black coloration, there is often the question of a possible maximum dosage. In practice, dosages of up to 5 % are usually recommended and in-house testing is advised. Higher dosages involve some risks such as insufficient resistance to frost and freeze-thaw cycles with de-icing salt. There is a growing demand on the market for a deep-black concrete coloration with a bluish cast, however, that cannot be achieved in practice with classic iron oxide in the usual dosages of up to 5 %. Figure 3 clearly shows that there exists an “aesthetic design color gap” between the color shades with black-bluish cast achieved with the 3 % minimum dosage of a carbon pigment and the yellow-tinted anthracite achieved with conventional, classic iron oxides in realistic dosages of 5 %.
Achieving black and deep blue-black anthracite color shades
As a result of the significant differences in the optical performance of iron oxides and carbons, efforts have been made for years to develop modern and more efficient iron oxides with more coloring strength and, most importantly, diminished yellow tint in order to close the “aesthetic design color gap”. This has led to a significant expansion of the range of black iron oxides in recent years.
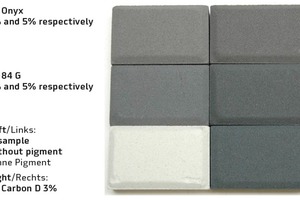
Figure 9 shows a classic GF Onyx iron oxide and the newly developed GF 84 G in dosages of 3 % and 5 % respectively. The image clearly shows the increase in coloring strength achieved in comparison to a high-quality carbon in facemix quality with the recommended minimum dosage of 3 %.
The modern iron oxides enable richer shades of black to be achieved and allow the production of the increasingly popular anthracite color tints which appear to be bluish-gray rather than yellowish.
The specific production of anthracite tints, whether as an elegant, anthracite-gray single color or incorporated in color-mix concrete blocks or so-called “coquina shades”, is in keeping with current trends. Carbons should not be used for this purpose because there is no sufficient resistance to weathering at low dosage levels (< 3 %). It is recommended to examine the use of white cements or mixtures of white and gray cements as binder in the production of these anthracite products. The reason for this is that gray cements may often cause unwelcome gray-greenish to yellowish color portions in the concrete products and do therefore not meet aesthetic requirements (Fig. 10). There is a very strong desire for an elegant “bluish-gray” tint that in some cases could only be met by adding rather high-priced inorganic blue pigments. It is here that modern blue-nuanced iron oxides provide both alternative solutions and saving potentials.
Mixtures of black iron oxide and carbon pigments
Considering the excellent coloring properties of carbon pigments and generally outstanding resistance of iron oxides, it is no surprise that mixtures of iron oxides and carbons are offered on the market. Even though these combinations provide excellent coloring strength, their use requires close examination with a very critical eye. The coloring strength is determined to a disproportionate extent by the carbon component. The use of very coarse-particle carbons by the color supplier enables excellent levels of resistance to be achieved. It must be taken into account, however, that there is only a certain proportion of carbon present so that the reserve dosage for the carbon portion described above is not achieved in these mixtures. As a typical example, a formulation containing 80 % of iron oxide and 20 % of coarse-particle carbons (GF Karat) is shown in Figure 11. As anticipated, the coloring strength achieved ranges between that of high-quality iron oxides and coarse-particle carbon pigments.
Problems arise with mixtures that contain fine-particle carbons of high coloring strength and iron oxides of low quality and low coloring strength. If weathering occurs in the carbon portion, only the low amount of basic coloration of the iron oxide portion remains. In real life, these products cannot but fail the quality test.
Summary
Both iron oxides and carbon pigments are perfectly viable. Taking specific advantage of their characteristic properties enables the raw material-related differences to be put to ideal use in solving the coloring tasks at hand. The boundary conditions are determined by technical and commercial requirements as well as aesthetic aspects.
A noticeably blacker degree of coloring can be achieved both with advanced black pigments and with iron oxides. In addition, these iron oxides enable anthracite color tints of different shades to be obtained that appear to be bluish-gray rather than yellowish. The blue-nuanced, deep-black coloring of concrete paving blocks that can be achieved with high-quality carbon pigments, however, cannot as yet be realized with iron oxides.