Insights into the Very Early Nucleation and Crystallization
C-S-H or calcium silicate hydrate presents the main hydration product of ordinary Portland cement (OPC). C-S-H is generated from the hydration of the dicalcium silicate (C2S) and tricalcium silicate (C3S) phases via a dissolution-precipitation mechanism and presents the binding phase which is responsible for the strength and durability properties in hardened cement.
Generally, C-S-H exhibits a low crystallinity and typically a Ca/Si molar ratio of ~ 1.6 - 1.8 in hardened cement. The layered structure of C-S-H consists of linear silicate chains which are aligned in “dreierketten” sequences and share oxygen atoms with calcium ions in the same plane.
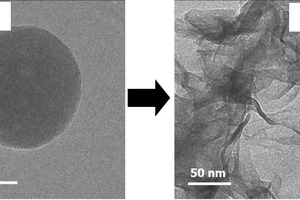
In order to be able to observe its very early nucleation, C-S-H was synthesized from Ca(NO3)2 and Na2SiO3 via the co-precipitation method. The early nucleation and crystallization of the synthesized C-S-H were observed via TEM imaging
After 5 minutes of reaction, pure C-S-H particles exhibit a globular morphology, with diameters in the range of ~ 40 - 60 nm. For the synthesized C-S-H, the transformation from a globular to a foil-like morphology had already started 15 minutes after the Ca(NO3)2 and Na2SiO3 solutions had been combined. After 1 hour, the C-S-H globules had completely disappeared while a network of C-S-H nanofoils with lengths of ~ 150 nm and a thickness of ~ 5 nm was found.
The globular precursor of C-S-H exhibits a highly disordered structure containing branched silicate chains. Whereas the C-S-H foils formed after the conversion show a layered structure of semi-crystalline C-S-H containing non-branched silicate chains.
This shows that synthetic C-S-H follows a non-classical nucleation mechanism where the morphology of the precursor significantly differs from that of the final bulk material.